Steady-state concentration (Css) occurs when the amount of a drug being absorbed is the same amount that’s being cleared from the body when the drug is given continuously or repeatedly (figure 1). Steady-state is achieved when the concentration of the drug in the body stays consistent.
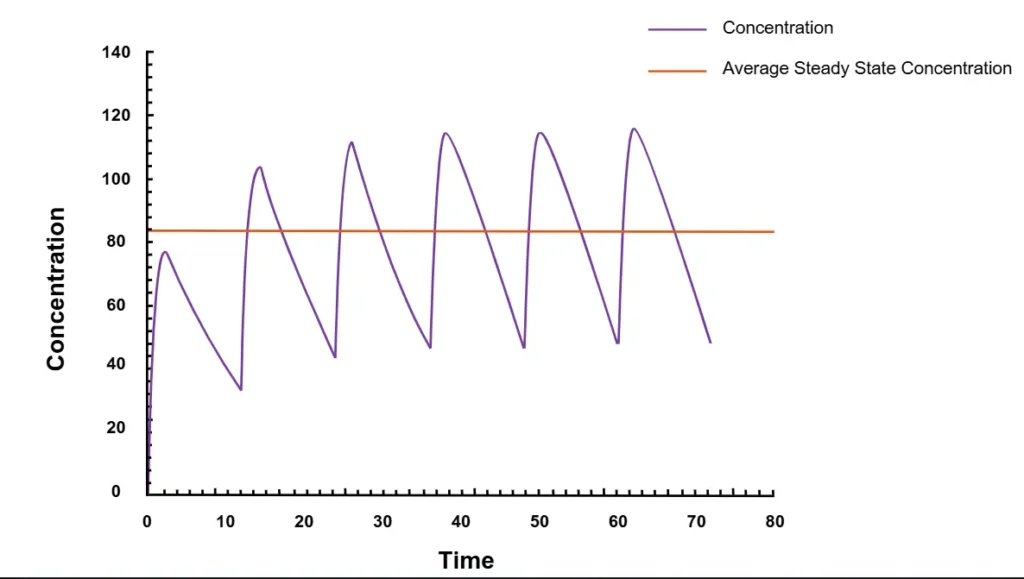
Here’s a good way to think about it: Imagine a coworker is absent, and she left a delicious box of chocolates in her office. You can’t resist, and you take two candies. The next morning, you replace the candies so your office mate will never be the wiser. But you later discover she’s out again, so you boldly take three chocolates, and replace those the following morning. As this continues, the chocolates are in a steady-state, meaning the number of candies doesn’t change over time. Whenever a chocolate is taken, it’s soon replaced in the box. The input rate is the same as the elimination rate. In pharmacokinetics (PK), those chocolates are drug molecules, and they’re being replaced at the same rate—through new doses—that they’re being removed from the body. Many drugs are designed to be administered repeatedly, therefore characterization of Css is often necessary in order to predict how a drug will behave in clinical settings.
Importance of Steady-State in Drug Development
Understanding steady-state is important for choosing the right dose and dosing interval to achieve a desired steady-state concentration—and for determining how long it will take for therapeutic exposures to be achieved during repeat or continuous dosing since it might take several doses for a drug to achieve therapeutic benefit. Multiple ascending dose (MAD) studies are early phase studies which help to characterize the PK of a drug at steady-state. Additionally, in studies conducted in special populations and in studies for assessing drug interactions, it may be necessary to assess whether PK parameters, including Css, change based on demographic factors or extrinsic factors such as drug-drug interactions.
Factors That Affect Steady-State Concentration
Average Css is an important parameter during the steady-state and it can be calculated as the total exposure (AUC) over one dosing interval (𝜏):
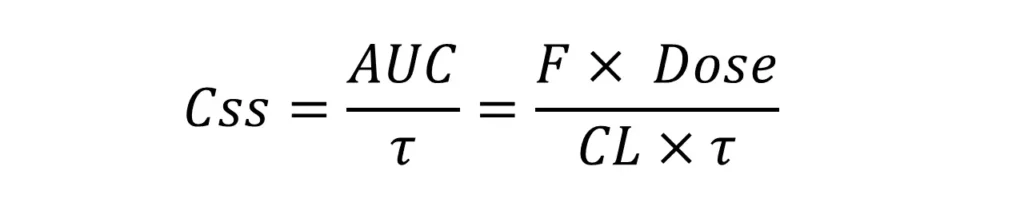
Thus, Css can fluctuate depending on many factors, such as:
- Dose: Amount of a drug to be taken one time.
- Dosing interval: The time interval between giving doses.
- Drug clearance (CL): The rate (speed) at which a drug is eliminated from the body.
Dose
Higher doses will increase the Css values, and lower doses will decrease them. The dose required to reach and maintain steady-state depends on the drug clearance rate, which in turn, can be affected by the patient’s demographics.
Dosing interval
The dosing interval affects steady-state concentration in an inversely proportional way. The shorter the dosing interval, the higher the steady-state concentration values.
Drug clearance
In general, the slower the clearance, the more of the drug will remain in the system and the higher the Css (and vice versa). A patient’s drug clearance rate, and therefore the Css, can be altered by demographic factors. A patient’s weight, their excretory and metabolic functions, and other drugs they’re taking can all cause fluctuations. For example, if someone with renal failure is administered a drug that’s eliminated mostly via the kidneys, the steady-state concentration of that drug will be higher than it would be for someone with healthy renal function who’s getting the same dosage.
Half-life and Steady-State Concentration
For most drugs, the time to reach steady-state is four to five half-lives if the drug is given at regular intervals—no matter the number of doses, the dose size, or the dosing interval. A half-life is how long it takes for half of the drug to be eliminated from the body. For simplicity, let’s assume we administer one dose every half-life. If a single dose is given every half-life, half of the first dose will be cleared from the body before the next dose. So, after the second dose, there will be 1.5 doses in the body. Half of that is eliminated, then the next dose is given, meaning there are now 1.75 doses in the body. By the time dose #6 (after five half-lives) is administrated, there will be close to two doses in the body, which means one entire dose is eliminated with each dosing interval. If we continue dosing at the same frequency as every half-life, the amount we dose will be eliminated during each dosing interval. As a result, drug concentrations in the body remain constant (steady).
Another way to think about steady-state:
- After Dose 1: There are 0.5 doses left at the end of the dosing interval. This means we’re at 50% steady-state.
- After Dose 2: There are 1.5 doses in the body, then half is eliminated to leave 0.75 doses (75% steady-state).
- After Dose 3: There are 1.75 doses in the body, then half is eliminated to leave 0.875 doses (88% steady-state).
- After Dose 4: There are 1.875 doses in the body, then half is eliminated to leave 0.9375 doses (94% steady-state).
- After Dose 5: There are 1.9375 doses in the body, then half is eliminated to leave 0.96875 (97% steady-state).
At 97%, we’re considered to be at approximate steady state, where the rate of input equals the rate of elimination at one dose per dosing interval.
Loading Dose and Steady-State Concentration
For a drug with a short half-life, steady-state is achieved rather quickly. If you have a drug with a long half-life and a patient who needs to achieve a therapeutic effect fast—for example, a critical care patient who needs antibiotics—how can you get that effect without having to wait days or weeks? The bad news is that it always takes the same amount of time to achieve steady-state: Four to five half-lives. The good news is that you can still achieve a therapeutic effect more quickly with a loading dose. A loading dose is a higher dose administered on treatment initiation. It will still take the usual four to five half-lives to reach steady-state, but the initial concentration will be closer to the eventual steady-state concentration—which means the therapeutic effect will happen faster.
Conclusions
Because many drugs are administered on a repeated basis, steady-state concentration (Css) is an important PK parameter to characterize in certain drug studies. Understanding a drug’s Css is critical in selecting an appropriate dose and dosing frequency to achieve safe, therapeutic drug concentrations in patients.
Are you interested in learning how Allucent’s PK experts can assess steady-state concentration in your drug development journey? Contact us today to speak with a member of our pharmacokinetics A-team.
