Problem
A client conducted a clinical study, which included an assessment of their drug’s effect on QT prolongation. However, it then became necessary to conduct a thorough QT (TQT) study to further characterize the risk of QT prolongation at higher doses of the study drug.
Background
Cardiac safety assessments are critical for drugs in development because some compounds delay cardiac repolarization, resulting in QT interval prolongation that may cause potentially fatal arrythmias. For many drugs, the potential for pharmacologic prolongation of the QT interval involves performing a standalone TQT study. In TQT studies, collection of pharmacokinetic (PK) data at the proper timepoints allows us to see if drug exposures are associated with the level of QT prolongation (or lack thereof) observed during the study.
Methods
Allucent provided input for the overall design of the TQT study (e.g., determining the use of parallel groups versus crossover) and assisted with selection of the maximum dose needed to cover potentially high drug exposures that could occur clinically, such as increased exposures due to drug-drug interactions. The doses chosen needed to be high enough to allow for sufficient exposures, while at the same time not so high that they would impact the safety of study participants. Allucent helped select doses by drawing on existing PK data for the drug and taking safety into consideration. Safety features incorporated into the study included use of sentinel subjects and safety data review before proceeding to higher doses. Allucent also confirmed that PK timepoints were properly matched to electrocardiogram (ECG) collections and assessed the value of using a positive control (moxifloxacin). The final design provided a balance between safety and adequate PK exposures to allow for assessment of the exposure-response relationship for QT prolongation.
Outcome/Solution
The client utilized Allucent’s input into the study design and the study was successfully completed. Drug exposures were sufficient to properly characterize the exposure-response relationship. The drug was well tolerated and no clinically meaningful effect on the corrected QT interval was observed following administration of the studied doses (Figure 1). Therefore, this study was a negative TQT study as described in the ICH E14 clinical guidance, which provided confirmation of minimal QT prolongation potential of the investigated drug.
About Allucent
We have the same mission as you: To develop and deliver essential medicines to the patients who need them. We do this through deep collaboration to understand your needs, increasing your confidence in decision making and delivering innovative strategies. By translating complex data into actionable insights that informs dosing, study designs, and modeling, the Allucent Clinical Pharmacology, Modeling & Simulation team can help deliver on your next breakthrough.
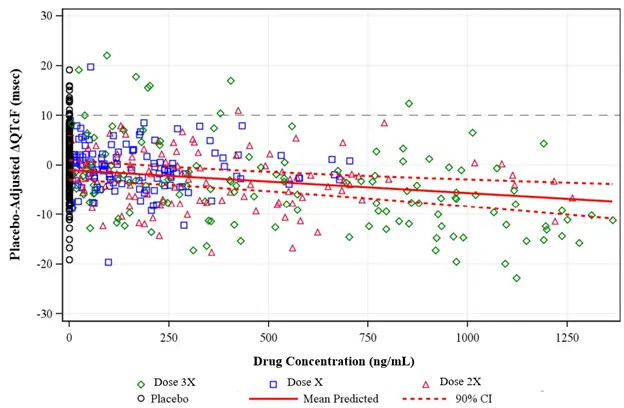
ΔQTcF =change-from-baseline corrected QT interval using Fridericia’s formula; CI=confidence interval.
Reference:
Rozakis R, Sevinsky H, Ham R, et al. (September 8-10, 2024). Phase 1, Single-Center, Randomized, Placebo-Controlled, Partially Blinded, Single Ascending Dose Study on the Effects of Troriluzole on corrected QT in Healthy Subjects. American College of Clinical Pharmacology, Bethesda, Maryland, United States.