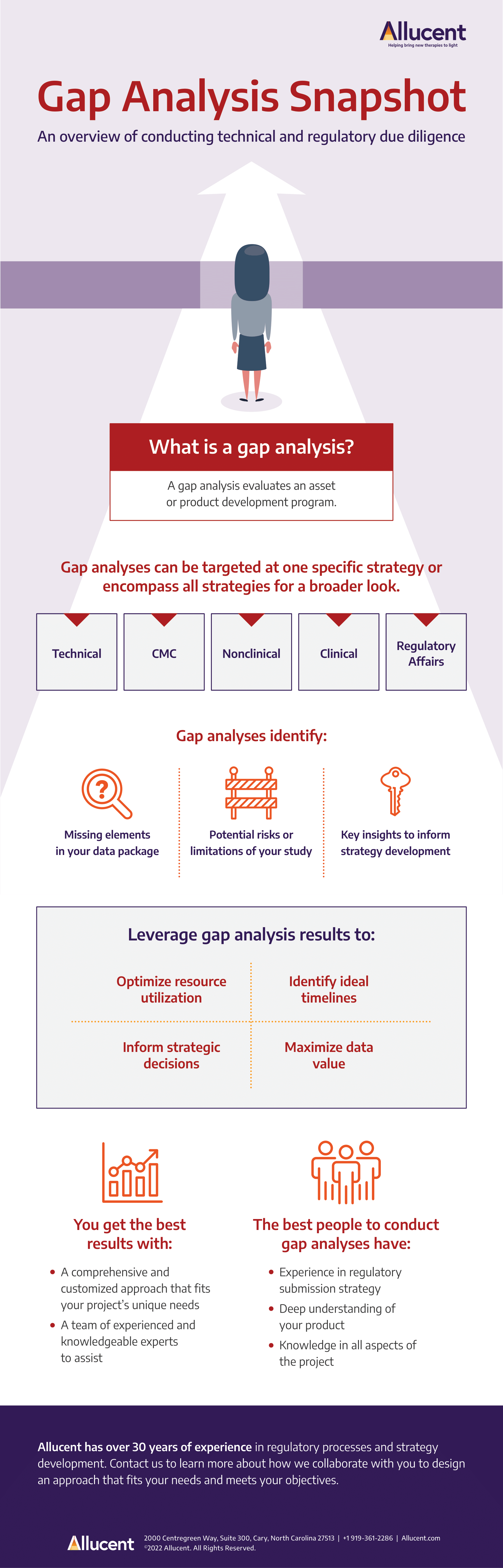
When building a successful regulatory strategy, it is critical to keep the end objectives in mind and design a process that is tailored to your specific product. Planning for regulatory requirements can help you avoid non-compliant practices from the very beginning, but how do you make sure your strategy is comprehensive? The answer may be conducting a gap analysis.
Gap analyses evaluate your development program from a technical or regulatory perspective using critical documents, data, and information. Conducting a thorough analysis requires a depth of expertise and knowledge in many areas, including scientific, technical, CMC, nonclinical, clinical (strategy and pharmacology), and regulatory affairs.
Learn more about the benefits of a gap analysis in this snapshot view.