Background and Problem
Allucent’s client required assistance using modeling and simulation to determine the feasibility and design of a First-in-Human (FIH) study for their program. The client’s drug was created to inhibit an enzyme responsible for neurogenerative and neuroinflammatory pathways in the brain. The pharmacodynamic (PD) objective associated with the drug was inhibition of the enzyme of interest ≥ 90% in the brain over a steady-state dosing interval. However, there were conflicting in vitro data on brain penetration, as well as toxicity findings in high dose animal studies that appeared to be associated with the maximum observed concentration (Cmax). Based on the toxicity findings, a Cmax limit for clinical exposure was defined, however it was not clear if it was possible to achieve the client’s PD objective while adhering to this limit. That is where Allucent came in — to provide FIH study design expertise and to help determine how much of the drug should be given to achieve the clinical PD objective, while simultaneously ensuring participant safety.
Our Solution
Allucent built a physiologically-based model to predict human pharmacokinetics (PK)/PD based on the client’s preclinical PK and pharmacology data and physiological parameters. The model was verified with independent data not used in the model development. Using the model, predictions were made for PK in the plasma as well as specific central nervous system compartments including the cerebrospinal fluid (CSF) and inside the brain (where drug would need to be delivered to be effective). The CSF levels of the drug were found to be equivalent to the free drug concentration in blood. Allucent then simulated clinical inhibition of the enzyme of interest in a healthy participant population. At the doses simulated, the model additionally showed exposures within the Cmax safety limit, confirming the feasibility of a Phase 1 FIH study in healthy participants.
Outcome
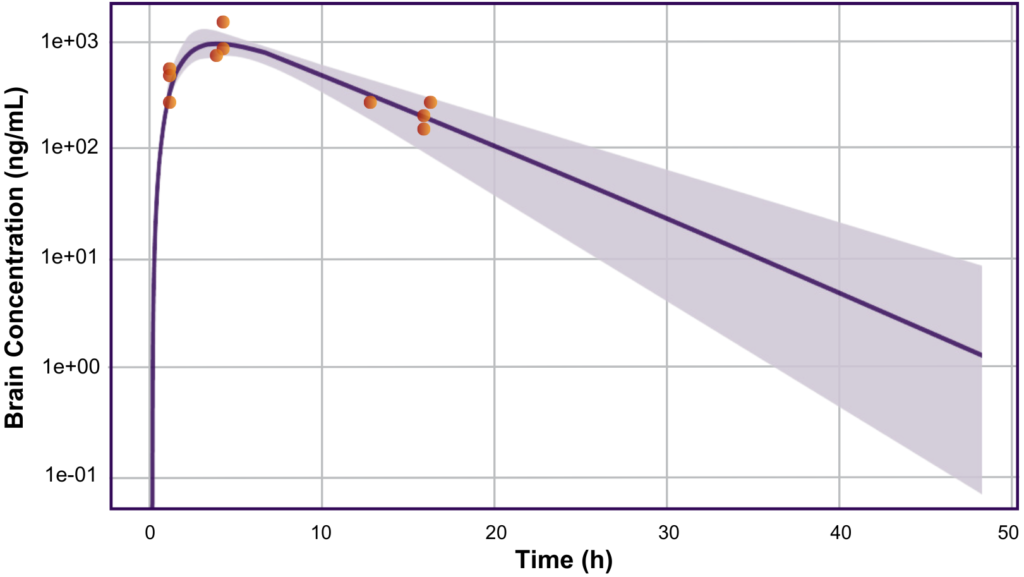
Based on the simulations and predictions from the model, Allucent developed the FIH protocol design for the client, including the selection of doses. When the data became available from the study, it was found that the drug had readily penetrated the CSF and was inhibiting the enzyme of interest in the brain. Exposures (Cmax) of the drug were within the appropriate limits and were accurately predicted within 2-fold of the actual PK results by the model (Figure). The drug was confirmed to be well tolerated in the healthy participant population with no severe adverse events and very few adverse events overall, and the model was optimized to inform future decisions in the clinical program.
Modeling and Simulation Services
For neurotherapeutics, implementing an integrated nonclinical development plan is needed to effectively support clinical development. Model-informed approaches are valuable in incorporating diverse data for translation into clinical decision-making and study designs.
Allucent has an expert team with nonclinical and clinical expertise in PK, PD, and model-informed drug development (MIDD) to guide your program’s decisions. As part of the services provided by Allucent, our team will create a custom, model-based drug development plan that will facilitate the selection of the most important model for your compound and disease area.
Contact us today to learn how Allucent’s modeling and simulation services can help you gain insights that move your clinical development program forward.
