Case Study
Background and Problem
Understanding the concentration of drugs in the central nervous system (CNS) is crucial for effective drug development. Traditionally, cerebrospinal fluid (CSF) drug concentrations have served as a surrogate for measuring drug levels in brain tissue, especially for drugs targeting the CNS. Lumbar punctures are commonly employed in clinical studies to assess CSF drug concentrations, providing valuable insights into the drug’s efficacy. Our client wanted a better understanding of systemic drug concentrations to lumbar CSF concentrations of their investigation product.
Our Solution
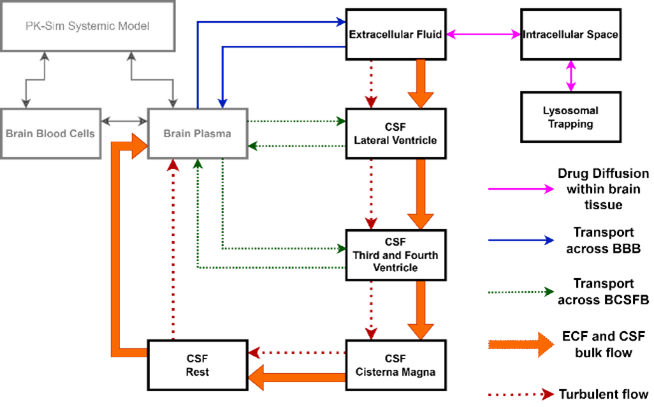
To address their questions, we integrated a comprehensive physiologically-based pharmacokinetic (PBPK) CNS model developed by Yamamoto et al 2017 with a whole-body PBPK model. This integration allowed for prediction of both systemic and CSF drug concentrations. The extended CNS PBPK model includes 4 CSF
compartments, a lysosomal compartment to predict any pH intracellular lysosomal trapping of the drug, and accounts for transport across the blood-brain barrier and blood-CSF barrier. The model was model was validated against published data of CNS-penetrant drugs (see figure).
Related Blog: Managing Neurotoxic Risks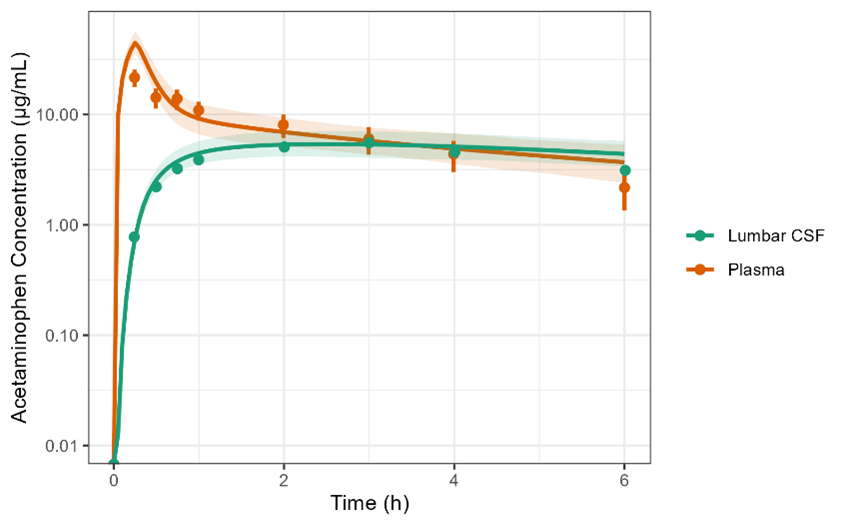
Outcome
The extended CNS PBPK model successfully predicted both plasma and lumbar CSF concentrations of the client’s study drug with great precision. This model can be easily reparametrized to allow for predictions in rats which would be helpful in preclinical studies.
Related Case Study: Power of Predictive Modeling
Modeling and Simulation Services
For neurotherapeutics, implementing an integrated nonclinical development plan is needed to effectively support clinical development. Model-informed approaches are valuable in incorporating diverse data for translation into clinical decision-making and study designs.
Allucent has an expert team with nonclinical and clinical expertise in PK, PD, and model-informed drug development (MIDD) to guide your program’s decisions. As part of the services provided by Allucent, our team will create a custom, model-based drug development plan that will facilitate the selection of the most important model for your compound and disease area.
Contact us today to learn how Allucent’s modeling and simulation services can help you gain insights that move your clinical development program forward.
