Allucent has extensive experience across multiple aspects of decentralized trials including both fully virtual and hybrid approaches. Hybrid and Decentralized Clinical Trials (DCTs) offer great potential to rethink and refresh the traditional clinical trial models, enabling patients to interact with clinical researchers away from the traditional site setting. These approaches can reduce the patient burden of participation and have the potential to increase access to a wider geographic footprint and, thus, a more diverse patient population.
Allucent’s Patient Direct Trials (PDT) offering is a customized decentralized trial solution that’s puts patients first and supports sites to effectively manage data collection and patient engagement. One advantage of Allucent’s PDT is that it can utilize an industry leading platform, an all-in-one solution that enables our clients to offer a patient and site-centric experience while collecting the data necessary for their drug development. Other important components of the platform is the inclusion of home health, concierge, and IMP supply through our partners making participation it easier for patients and their families, thereby increasing the healthcare options for participants.
Advantages of Decentralized Clinical Trials
- Enhanced Patient Access and Participation
- Improved Patient Engagement and Retention
- Accelerated Recruitment
- Enhanced Data Quality and Real-Time Monitoring
- Patient Safety and Comfort
- Environmental Impact
- Cost Efficiency
Allucent Patient Direct Trials
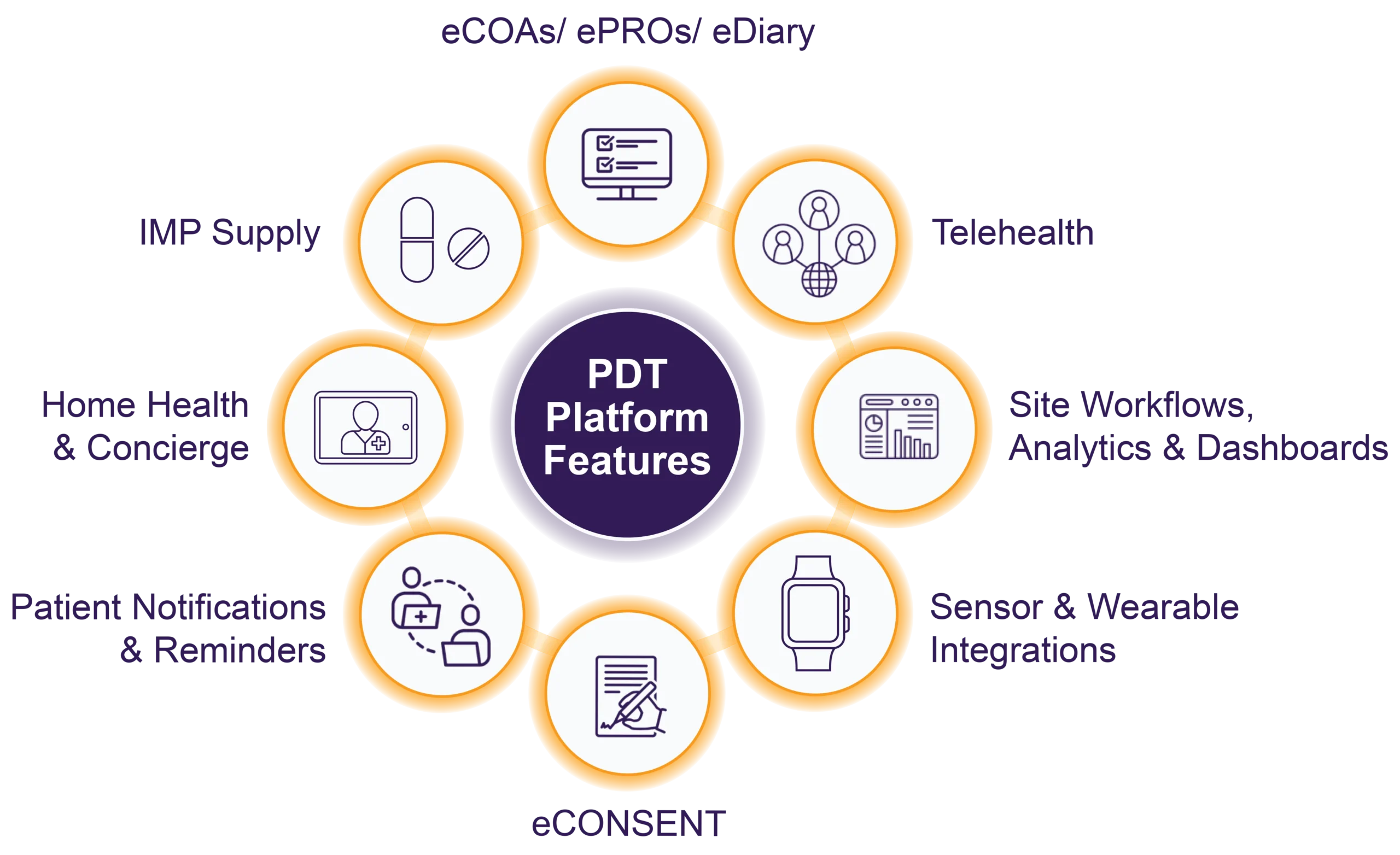
ECOA


Electronic clinical outcome assessment (eCOA) is key to DCTs, but compliance and data quality are vital. With a unified, secure, continual data flow from multiple sources, compliance and protection of study data are assured.
Telehealth


Use of telemedicine retains the human touch while reducing the burden of trial participation on patients and clinicians alike.
Site Data Capture & Analytics


Thanks to state-of-the-art technology, data capture on-site, remotely, or during telehealth virtual visits is streamlined. Real-time data on study performance, as well as predictive insights on study completion, enrollment, and retention, are delivered via a dashboard, helping drive decision-making and optimize outcomes.
Sensors & Wearables


Simplicity in using wearables and medical devices delivers a trial experience for participants that increases their engagement while simultaneously supporting robust data collection for exploratory, secondary and even primary endpoints.
eConsent


Compliant, efficient eConsent can reduce human error and site burden while offering patients’ convenience. Participant consent goes beyond the eSignature to include education and engagement ensuring participants have the information they need. It can be managed securely, including both single and dual signatures, on any device and will always be deployed in line with local regulatory guidelines.
Patient Notifications & Reminders


Manage patient interactions throughout the trial through direct notifications and reminders ensuring participants are well-informed, engaged, and compliant with the study requirements, which in turn supports the trial’s success.
Home Health


Our partners support us in delivering clinical trials where patients live and work, making participation easier for patients and their families and increasing the healthcare options for participants. Data can be collected via eSource directly into our PDT platform allowing sites to visualize the data in almost real time.
IMP Delivery


We partner with organizations to support the direct delivery of IMP, test kits, and other clinical supplies directly to the participants’ homes and keep participants and sites engaged and informed via our PDT platform.
Other Areas of Expertise
Additional Resources

News
Allucent Receives BARDA-Funded Project NextGen Award to Support a COVID-19 Vaccine Decentralized Clinical Trial
Learn More

Webinar
Cell and Gene Therapy Clinical Trials: How to Successfully Operationalize a Trial of Prolonged Duration
Learn More
Fact Sheet
Patient Engagement Fact Sheet
Learn More



